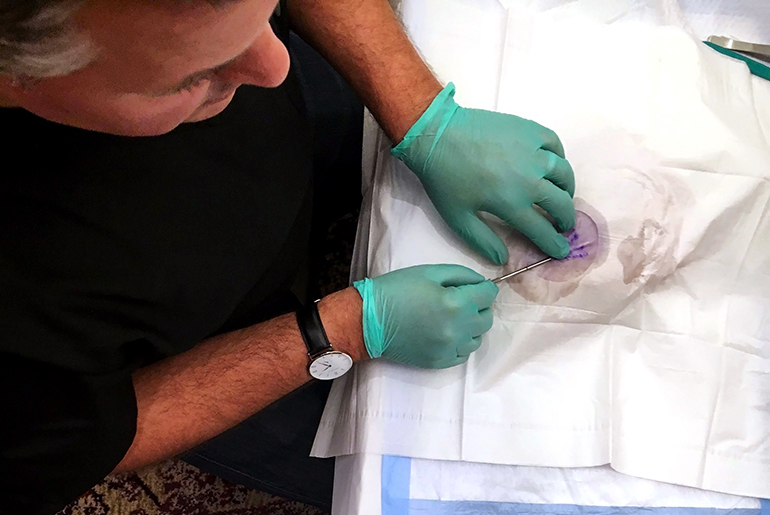
In a big hotel conference room near New York’s Times Square, six doctors huddle around a greasy piece of raw pork. They watch as addiction medicine specialist Michael Frost delicately marks the meat, incises it and implants four match-sized rods.
“If you can do it well on the pork, you can easily do it on the person,” Frost tells his audience.
Frost consults for Braeburn Pharmaceuticals, the company behind the newly FDA-approved treatment Probuphine, and is teaching doctors how to use it. They are learning to implant it in pork so they can later implant it in patients’ arms.
Although addiction specialists welcome Probuphine, which delivers a constant dose of the drug buprenorphine over six months, at this early stage it’s complicated for physicians to add it to their repertoire. Because physicians who treat addiction don’t necessarily have experience with surgery or access to sterile spaces, some are having to learn a new skill and develop new systems.
Probuphine is unlike any other addiction treatment on the market. It promises to be life-changing for people already stable in recovery using medication-assisted treatment, who would otherwise need a daily dose of a similar drug to stay free of cravings and withdrawal pains.
Patients using Probuphine were 14 percent more likely to stay opioid-free compared to those using a daily sublingual version of buprenophine, according to a study published in July in JAMA, the Journal of the American Medical Association. Patients in this study had been stable on buprenorphine for an average of three and a half years beforehand. The authors do caution against generalizing these findings. Most participants, they note, were white, employed, had at least a high school education and were previously addicted to prescription opioids rather than heroin. Still, clinicians see the new treatment’s potential.
“They don’t have to be dependent on taking something every day. It takes the choice out of that,” said Ella Leers, a doctor who treats substance abuse at the Carnegie Hill Institute in Manhattan.
The FDA approved Probuphine under the condition that physicians are trained and tested before implanting or even prescribing the treatment. There are three kinds of certification: implanter, prescriber or both. If doctors can’t perform the implanting themselves, they need to coordinate with another doctor who can.
To date, over 1,800 healthcare practitioners have been certified — 27 have implanted dozens of patients, according to a representative for Braeburn Pharmaceuticals, Probuphine’s maker.
Gloria Baciewicz, chief of addiction psychiatry at the University of Rochester Medical Center, said using the new treatment will take some adjustments. But, she added, there need to be as many effective treatments as possible for opioid use disorder.
“Now with Probuphine, we have to take it up to a whole different level because we have to have either agreements with implanters or a room where we can implant. We have to get the equipment. There will be a lot more to do,” she said. Her team was already planning on moving to another space, which will have the facilities they need to conduct minor surgery.
Prescribing Probuphine may also call for a new approach to the counseling and behavioral therapy that is typically recommended for those on medication-assisted treatment.
“If you’re implanting something that can be there for six months, you want to make sure that the patients are still coming in to get the other types of support that they can use because of their addiction issues,” said Leers.
There are also questions about insurance coverage. Billing codes are still being established. For now, doctors need to buy the Probuphine kits that run almost $5,000 themselves, and then bill patients or insurance companies.
Braeburn has offered to help physicians verify if an insurance plan would reimburse any of the cost. According to the company, Blue Cross Blue Shield and United Healthcare approved reimbursement for a few patients who have implants. Medicare, Medicaid and the VA have Probuphine in their formulary and are required to cover it if deemed medically necessary.
Despite having to get certified and the other hurdles, many doctors welcome the treatment option. Opioid drug overdoses have reached epidemic levels — roughly 78 Americans die every day from opioid overdose, according to the Centers for Disease Control and Prevention.
So it’s good to have a another way to deliver medication-assisted treatment, said Richard Rosenthal, medical director of addiction psychiatry for the Mount Sinai Health System. Rosenthal was one of two principal investigators on a Probuphine clinical trial.
“Everybody is waking up to the fact that we’re in the midst of an opioid epidemic,” said Rosenthal. “There are actually very few medications for addiction of any kind. Given the addiction treatment system in the United States, most of the treatment that’s given is psychosocial. There’s very little use of FDA-approved medications.”
Probuphine made a difference, said Scott Jernigan of Jacksonville, Fla. He was in recovery for almost a year, taking another medication, when he signed up for a Probuphine clinical trial. He said Probuphine freed him from weekly doctor visits and pharmacy runs, and from fears of how sick he’d feel if he missed a dose or forgot to take his medication.
“[It] meant that I could become more of what my normal is going to be,” Jernigan said.
Some specialists recommend patients stay on medication-assisted treatment for years, or even indefinitely. For now, Probuphine can only be prescribed for two runs of six-month use and is meant for people already stable on 8 mg or less of a medication like buprenorphine.
Authors of the JAMA study suggest further investigation of Probuphine to gauge issues often associated with buprenorphine, such as diversion or pediatric exposure.
This story is part of a reporting partnership with NPR, Side Effects Public Media and Kaiser Health News.
This story was produced by Kaiser Health News, an editorially independent program of the Kaiser Family Foundation.